Allos announced today that it has held a meeting with the U.S. Food and Drug Administration (FDA) to optimize the design of their Phase 3 trial.
Our global team is dedicated to improve the lives of patients with Fragile X Syndrome and Autism Spectrum Disorders
ABOUT US
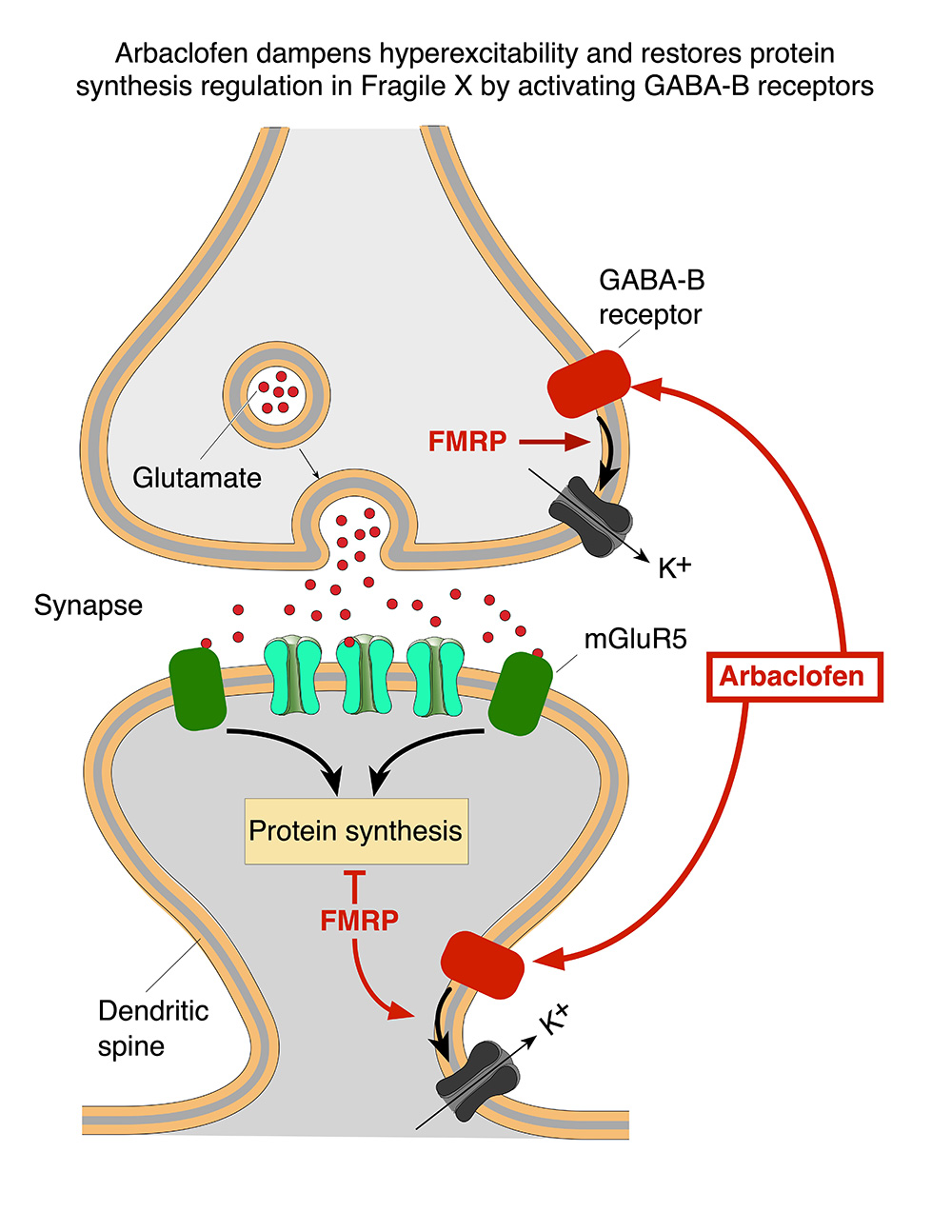
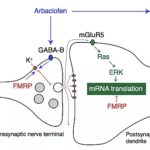
We are a global team dedicated to improve the lives of patients with Fragile X Syndrome and Autism Spectrum Disorder. Our work spans three decades of research in neuroscience and intellectual disabilities. The “mGluR theory of fragile X” proposed by MIT Professor Mark Bear and collaborators in 2004 is widely considered to be a key conceptual breakthrough, and laid the foundation for development of disease-modifying therapies for treatment of Fragile X Syndrome and Autism Spectrum Disorder. Allos Pharma is committed to develop and market the first approved therapy in Fragile X Syndrome.
The name Allos Pharma is derived from
allistic, meaning not autistic.
Disease Area
Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet A. 2014;164A:1648–58
Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci. 2012;35:417–43.
Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J,et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–90.
Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am J Med Genet A. 2014;164A:1648–58
Bhakar AL, Dolen G, Bear MF. The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci. 2012;35:417–43.
Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, Kronk R, Delahunty C, Hessl D, Visootsak J,et al. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–90.
SCIENCE
Founders

Mark Bear, PhD, Professor
Dr. Mark Bear is Picower Professor of Neuroscience in The Picower Institute for Learning and Memory and the Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology. He was an Investigator of the Howard Hughes Medical Institute for 22 years from 1994 to 2015 and served as Director of the Picower Institute from 2007 to 2009. Prior to moving to MIT in 2003, Dr. Bear was on the faculty of Brown University School of Medicine for 17 years.
Dr. Randy Carpenter’s career experience includes providing primary care to underserved populations, performing clinical and basic science research, and leadership positions in academic medicine, pharmaceutical, and biotechnology companies. Most recently, he founded Seaside Therapeutics to leverage breakthrough scientific discoveries and develop therapeutics capable of correcting molecular perturbations that increase vulnerability to intellectual disability and autism.

Randall Carpenter, MD
Fragile X Syndrome:
National Fragile X Foundation – www.fragilex.org
FRAXA Research Foundation – www.fraxa.org
RESOURCES
Autism Spectrum Disorder:
Autism Speaks – www.autismspeaks.org
Autism Science Foundation – autismsciencefoundation.org
SFARI – www.sfari.org
Selected publications
-
The mGluR theory of Fragile X mental retardation M.F. Bear, K. M. Huber and S.T. Warren, 2004 July. Trends in Neuroscience; 27(7): 370-377
-
The Autistic Neuron: Troubled Translation? R.J. Kelleher and M.F.Bear, 2008 Oct. Cell. 135(3): 401-6
-
Arbaclofen in fragile X syndrome: results of phase 3 trials E. Berry-Kravis, R. Hagerman, J. Visootsak, D. Budimirovic, WE. Kaufmann ,M. Cherubini, P. Zarevics, K. Walton-Bowen, P. Wang,M. Bear MF9, R. Carpenter, 2017 June. Journal of Neurodevelopmental Disorders; 12;9:3
- Arbaclofen produces clinically meaningful improvements in individuals with Fragile X: R. L. Carpenter, M. E. Matthiesen, D. C. Stoppel, S. Hudgens, M. F. Bear, 2022 July. NFXF International Fragile X Conference.

- Arbaclofen in Children and Adolescents with Autism Spectrum Disorder: A Randomized, Controlled, Phase 2 Trial.Veenstra-VanderWeele JM, Cook EH, King BH, Zarevics P, Cherubini M,Walton-Bowen K, Bear MF, Wang PP, Carpenter RL. Neuropsychopharmacology. 2016, 1-9 Oct 17. doi: 10.1038/npp.2016.237 [Epub ahead of print]
- STX209 (Arbaclofen) for Autism Spectrum Disorders: An 8-Week Open-Label Study. Erickson CA, Veenstra-Vanderweele JM, Melmed RD, McCracken JT, Ginsberg LD, Sikich L, Scahill L, Cherubini M, Zarevics P, Walton-Bowen K, Carpenter RL, Bear MF, Wang PP, King BH. J Autism Dev Disord. 2014 44(4):958-64.
- Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: A randomized, controlled, Phase 2 trial. Berry-Kravis E, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Gonzales-Heydrich J, Wang P, Carpenter RL, Bear MF, Hagerman R. Science Translational Medicine. 2012 19;4(152):152ra127.
- Reversal of disease phenotypes in the Fragile X mouse model by selective activation of GABA-B receptors.Henderson C, Wijetunge L, Nakamoto Kinoshita M, Shumway M, Postma FR, Brynczka C, Hammond RS, Rush R, Thomas A, Paylor R, Warren ST, Vanderklish PW, Kind PC, Carpenter RL, Bear MF, Healy AH. Science Translational Medicine. 2012 19;4(152):152ra128.
- R-baclofen reverses cognitive deficits and improves social interactions in two lines of 16p11.2 deletion mice.Stoppel LJ, Kazdoba TM, Schaffler MD, Preza AR, Heynen A, Crawley JN, Bear MF. Neuropsychopharmacology. 2018 Feb;43(3):513-524
- Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, Warren ST. Nat Chem Biol.2008 Apr;4(4):256-6
News
Allos announced presentation of “Arbaclofen produces clinically meaningful improvements in individuals with Fragile X” at the 18th NFXF International Fragile X Conference, July 14-17th, 2022.
Allos announced the exclusive license rights on arbaclofen in Fragile X Syndrome (FXS). The company is preparing the regulatory path for market authorization.
sign up for our newsletter
sign up with your email address to receive updates